difference between volumetric coulometric karl fischer titration inc|why are redox reactions important in karl fischer : store What is the Difference Between Coulometric and Volumetric Titration? Download PDF Copy. By Dr. Liji Thomas, MD Reviewed by . WEBCyberFile is a resilient file hosting that protects users from malicious and automated takedown requests.
{plog:ftitle_list}
WEBThe purpose of the JSON Sorter tool is to sort key names and key values, which can be both alphabetical and numerical values, and can be sorted in ascending or descending (reversed) order. JSON (JavaScript Object Notation) is a lightweight data-interchange format that is useful for exchanging data from client to server, or from server to client.
Karl Fischer (KF) titration is a specific moisture determination technique widely used by industry and scientists. The different ways of producing iodine can be divided into the coulometric method and the volumetric method.
What is the Difference Between Coulometric and Volumetric Titration? Download PDF Copy. By Dr. Liji Thomas, MD Reviewed by . This article takes a look at the difference between coulometric titration vs volumetric titration and provides some recommendations on . Coulometric Karl Fischer titration (KFC) is generally used for samples with a low moisture content between 0.001% to 1%. Volumetric Karl Fischer titration (KFT) is used for .
The main difference between the two techniques is how the water content is determined: volumetric KF titration measures the volume of titrant used, while coulometric KF titration .
The reaction between the Karl Fischer reagent and water leads to a color change, which is detected by an indicator, marking the endpoint of the titration. By accurately measuring the volume of the Karl Fischer reagent required to .METTLER TOLEDO's Karl Fischer titrators offer volumetric methods for water determination up to 100% and/or coulometric methods for low water content from 1 ppm to 5%. The titration vessel is made completely of glass which ensures exceptionally low drift, making the most accurate and precise results possible.THE BASICS OF KARL FISCHER TITRATION Karl Fischer titration is an analytical technique to measure the amount of water contained in various samples, which may be solid, liquid or gaseous. This method was originally developed in the 1930s by German chemist Karl Fischer. Karl Fischer titration is based on a 1:1 reaction between iodine and water in .
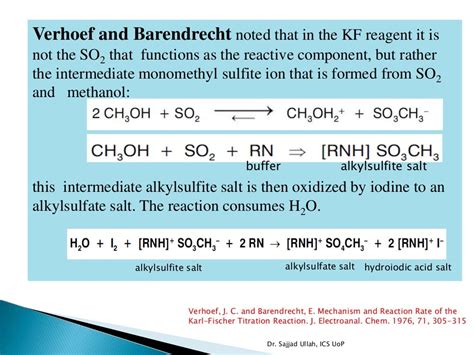
the volumetric determination of water and Hydranal-Coulomat AK, and Hydranal-Coulomat CG-K for coulometric determination (section 2.4). The range of KF titration reagents was completed with the addition of Hydranal-Water Standards (section 2.5), titration reagents for back titrations and buffering substancesThe technique was developed by a chemist named Karl Fischer. It is based on a reagent which reacts with water and converts the water into a non-conductive chemical. Karl Fischer provides for the specific detection of water content in a product. There are two methods used to perform the Karl Fischer titration test. One is known as Volumetric .
What are the differences between volumetric and coulometric Karl Fischer titration? The main difference between the two techniques is how the water content is determined: volumetric KF titration measures the volume of titrant used, while coulometric KF titration measures the amount of electricity used.
Karl Fischer titration is a titration method that uses volumetric or coulometric titration to determine the quantity of water present in a given analyte. This method for quantitative chemical analysis was developed by the German chemist Karl Fischer in the year 1935, Today, specialized titrators (known as Karl Fischer titrators) are available . CSC Scientific Company, Inc 2799-C Merrilee Drive Fairfax VA, 22031 800-621-4778 703-280-5142 (fax) [email protected] No part of this page or any other page in this site may be copied or reproduced in any way.What is the difference between volumetric and coulometric Karl Fischer Titration? The titrant can either be added directly to the sample by a burette (volumetry) or generated electrochemically in the titration cell (coulometry).Coulometric Karl Fischer Titration 1 Volumetric / Coulometric Titration Volumetric Karl Fischer Iodine is added by a burette during titration. Water as a major component: 100 ppm - 100 %
I recommend carrying out a volumetric or coulometric Karl Fischer titration using a certified water standard as sample. In volumetry, you can carry out a threefold titer determination followed by a determination of a different standard. Then, you can calculate the recovery of the water content determination of the standard.
why are redox reactions important in karl fischer
complexity. The KF titration is either volumetric for high amounts of water (100 ppm to 100%, Section 3.2)orcoulo-metric for low content of water (1 ppm to 5%, Section 3.1). The main difference between these techniques is that in the volumetric method, the amount of reagent consumed indicates the amount of water, while in the coulometric
What is the difference between volumetric and coulometric Karl Fischer Titration? The titrant can either be added directly to the sample by a burette (volumetry) or generated electrochemically in the titration cell (coulometry).The main difference between coulometric and volumetric Karl Fischer titration concerns titrant addition. In volumetric Karl Fischer titration, the titrant is added directly to the sample with a buret. Coulometric Karl Fischer titration features a generator electrode, which electrochemically generates the required iodine for the titration .Standard Test Method for Water Using Volumetric Karl Fischer Titration 1. . The 95 % limit for the difference between two such averages is 0.014 % absolute. 14.4.3 Reproducibility (Multilaboratory)—The standard deviation of results (each the average of duplicates), obtained by analysts in different laboratories has been estimated to be 0. .
What is the difference between volumetric and coulometric Karl Fischer Titration? The titrant can either be added directly to the sample by a burette (volumetry) or generated electrochemically in the titration cell (coulometry).
titration Coulometric KF titration Volumetric KF titration In practice small differences occur between the two methods which are displayed in the table. The advantages of the volumetry lie in the different types of sample adding and solvent variations, offering more fl exible operation potentials. Where on theThe main difference between coulometric and volumetric Karl Fischer titration concerns titrant addition. In volumetric Karl Fischer titration, the titrant is added directly to the sample with a buret. Coulometric Karl Fischer titration features a generator electrode, which electrochemically generates the required iodine for the titration . I recommend carrying out a volumetric or coulometric Karl Fischer titration using a certified water standard as sample. In volumetry, you can carry out a threefold titer determination followed by a determination of a different standard. Then, you can calculate the recovery of the water content determination of the standard.Coulometric karl fischer titration. The coulometric Karl Fischer titration has many benefits when the water concentration is as low as 10 μg. Coulometric Karl Fischer titration has the same chemistry but the main difference is the way of introducing iodine into the titration cell.
Karl Fischer titration is used to determine the water content of samples. It can be performed using either volumetric or coulometric measurement techniques.by using the coulometric and volumetric Karl Fischer titration instruments. Thanks to its selectiv-ity and precision, the Karl Fischer titration very easily and accurately established as the most important method for determining water and humidity. The basic principle of the water determination according to Karl Fischer (short: KF) is a reaction of This blog article explains the most important factors to consider in order to choose the optimal Karl Fischer titration technique: coulometry or volumetry. Coulometric Karl Fischer titration (KFC) is generally used for samples with a low moisture content between 0.001% to 1%. Volumetric Karl Fischer titration (KFT) is used for samples with higher levels of moisture .
What are the two types of Karl Fischer Titration? 1) Volumetric KFT In volumetric Karl Fischer, iodine is added mechanically to a solvent containing the sample by the . VOLUMETRIC SAMPLE SIZE COULOMETRIC SAMPLE SIZE 100% 0.02 to 0.05 g NOT RECOMMENDED 50% 0.05 to 0.25 g 0.01 g 10% (100,000 PPM) 0.25 to 0.50 g 0.01 to 0.05 g .The volumetric Karl Fischer Titration method is used to determine the water content accurately by adding iodine-containing titrants to the sample. The sample needs to be dissolved in an appropriate solvent to release the water completely. . Coulometric Karl Fischer Titration is preferred for liquid samples with very low water content . The potentiometric titrator and the Karl Fischer titrator are highlighted in this buyers’ guide to titrators. When choosing a KF titrator, the user should determine what types of solvents and samples will be utilized, and estimate the amount of moisture contained in the sample. Industry experts discuss the difference between the volumetric titrator and .The same chemical processesas in a volumetric KF titration take place , i.e.1 mol H,2O consumes 1mol of I . This results in a voltage difference between the Pt wires. The voltage drastically decreases in the presence of . Page 2 of 5 Instruments • Titrator with a mode for coulometric Karl Fischer titration . Electrodes Double Pt wire .
kf factor calculation by water
WEBAcompanhe os Resultados do Jogo do Bicho FEDERAL de 19 horas válido para todo o país, os sorteios são realizados as Quartas e Sábados as 19:00 de Brasília. Aproveite e .
difference between volumetric coulometric karl fischer titration inc|why are redox reactions important in karl fischer